Techniques
Optogenetics. Genetic or viral
approaches are used to express light-activated molecules in specific cells
(e.g. channelrhodopsin or archaerhodopsin) to define the role of
these neurons in circuits that mediate behavior and endocrine
responses. Such experiments are performed in vivo or (as done
here) in
vitro, where action potential firing is precisely suppressed by a
pulse of laser light.
viral
approaches are used to express light-activated molecules in specific cells
(e.g. channelrhodopsin or archaerhodopsin) to define the role of
these neurons in circuits that mediate behavior and endocrine
responses. Such experiments are performed in vivo or (as done
here) in
vitro, where action potential firing is precisely suppressed by a
pulse of laser light.
 Histology
and morphometry. We use many approaches to study the cellular
anatomy of the molecules, cells and circuits we study;
immunohistochemistry, phalloidin staining for actin filaments, antibody
staining of live or fixed cells, intracellular dye injections and
morphometric analysis of the surface area of single cells or neurons
imaged in live tissue slices. We also use Diolistic labeling of single
cells and various methods for tacing axonal pathways.
Histology
and morphometry. We use many approaches to study the cellular
anatomy of the molecules, cells and circuits we study;
immunohistochemistry, phalloidin staining for actin filaments, antibody
staining of live or fixed cells, intracellular dye injections and
morphometric analysis of the surface area of single cells or neurons
imaged in live tissue slices. We also use Diolistic labeling of single
cells and various methods for tacing axonal pathways.
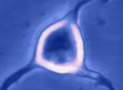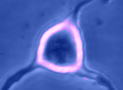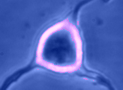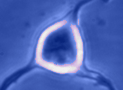 Digital
imaging. We perform many forms of imaging in live cells and
slices, including; dynamic analysis of changes in cell volume using
morphometric approaches; ion concentration imaging (e.g. Calcium) in
live cells or slices, fluorescence detection combined with
electrophysiology.
Digital
imaging. We perform many forms of imaging in live cells and
slices, including; dynamic analysis of changes in cell volume using
morphometric approaches; ion concentration imaging (e.g. Calcium) in
live cells or slices, fluorescence detection combined with
electrophysiology.
Tissue culture and molecular biology. Our experiments also involve the use of organotypic slice cultures, cultured cell lines, in-vitro transfection of these preparations, as well as standard molecular biology techniques such as western blotting and RT-PCR for detection of specific mRNA species in single cells and tissue extracts.
Electrophysiology. The Bourque Lab is first and foremost an electrophysiology lab. Most trainees will gain experience with many different electrophysiological techniques as required by their particular project, including:
Extracellular single-unit recording. Here, a glass pipettes filled with
saline solution is used to record action potentials fired by a neuron
at the tip of the pipette. The electrode is positioned using a micromanipulator.
What one sees on the oscilloscope or computer is shown at right. The
advantage of this technique is that the neuron is not damaged by
the recording process and one records the natural spontaneous electrical
activity of the cell in a relatively non-invase way. Recordings such as
these can last many hours.
with
saline solution is used to record action potentials fired by a neuron
at the tip of the pipette. The electrode is positioned using a micromanipulator.
What one sees on the oscilloscope or computer is shown at right. The
advantage of this technique is that the neuron is not damaged by
the recording process and one records the natural spontaneous electrical
activity of the cell in a relatively non-invase way. Recordings such as
these can last many hours.
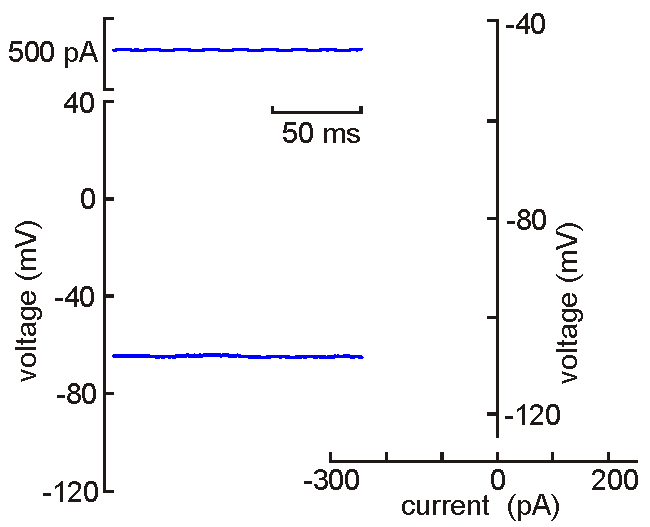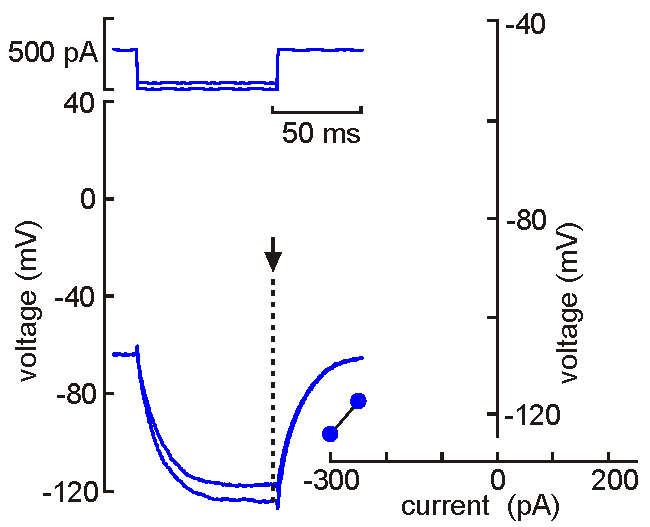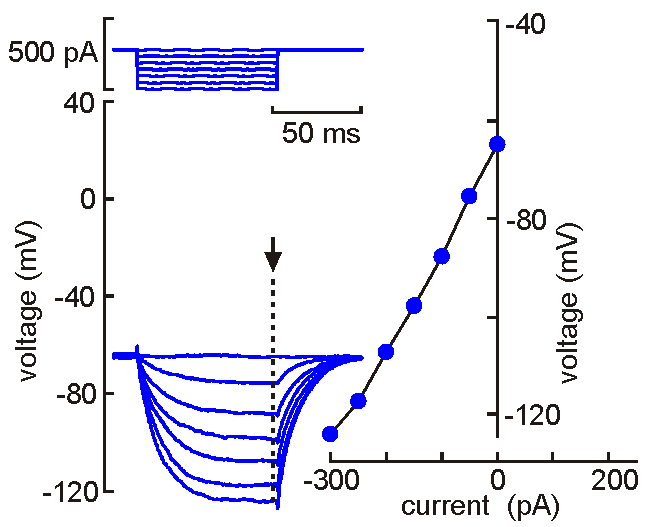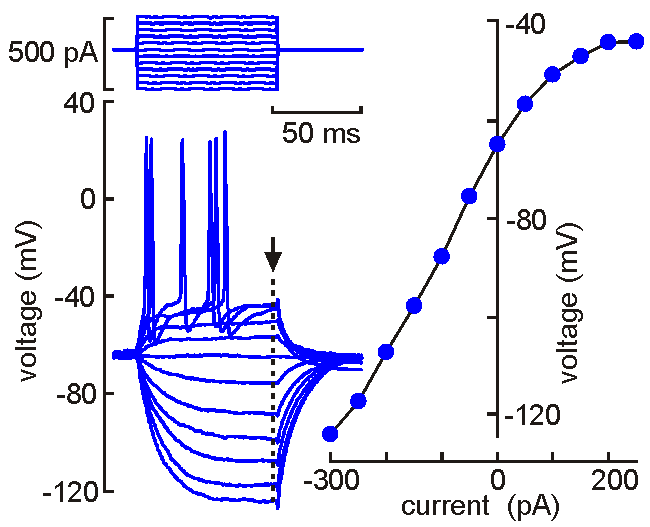 Intracellular
(sharp electrode) recording. This approach
uses a fine-tipped (~100 nm)
glass micropipette inserted into the neuron, allowing direct recording
of electrical events generated by the neuron (membrane potential, resistance, time
constant, synaptic potentials and action potentials). Current can be
injected to change the membrane potential of the cell (left). When the
neuron is depolarized past "threshold", action potentials are generated.
Voltage-current analysis can be performed by plotting voltage (at arrow)
as a function of current. The slope of the linear part of the curve
provides a measure of the input resistance of the cell. Although
this technique is relatively difficult to perform (compared with patch
clamp), sharp electrode recordings allow recordings to be obtained for
long periods without the common problems of dialysis and run-down that
affect neurons recorded by patch clamp.
Intracellular
(sharp electrode) recording. This approach
uses a fine-tipped (~100 nm)
glass micropipette inserted into the neuron, allowing direct recording
of electrical events generated by the neuron (membrane potential, resistance, time
constant, synaptic potentials and action potentials). Current can be
injected to change the membrane potential of the cell (left). When the
neuron is depolarized past "threshold", action potentials are generated.
Voltage-current analysis can be performed by plotting voltage (at arrow)
as a function of current. The slope of the linear part of the curve
provides a measure of the input resistance of the cell. Although
this technique is relatively difficult to perform (compared with patch
clamp), sharp electrode recordings allow recordings to be obtained for
long periods without the common problems of dialysis and run-down that
affect neurons recorded by patch clamp.
Unlike many other techniques, electrophysiological experiments provide data and information that can be observed in real time. No need to wait an hour or a day to know if the experiment worked! The animation shown above illustrates a measurement of the neurons' V-I profile as done in real time. Evidently protocols such as this are usually performed at a slightly slower rate, and must be repeated often during an experiment. But the information they provide can be interpreted on-line.
Patch clamp - single channel recording. Here, a larger tipped (diameter 1-2 microns) glass pipette is pressed against the membrane of a neuron while it is observed using a microscope. Gentle suction is applied and the high resistance seal that forms (a so-called "gigaseal") allows one to record the activity of individual ion channel proteins as the pore opens and closes to regulate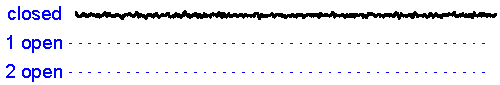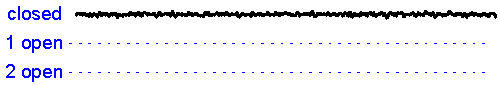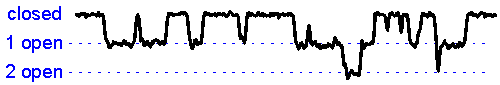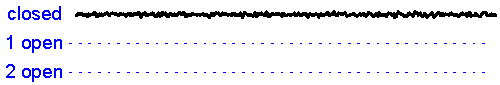 ther flux of ions (and thus
electrical current) across
the membrane. In the example shown at right we see several spontaneous openings of
a single channel. In one of the sweeps we see that two channels are opened
simultaneously, leading to a brief period of time when the current
recorded jumps
to total amplitude that is twice as large as that carried when a single
channel is opened (implying that there are at least two channels in the
patch!).
ther flux of ions (and thus
electrical current) across
the membrane. In the example shown at right we see several spontaneous openings of
a single channel. In one of the sweeps we see that two channels are opened
simultaneously, leading to a brief period of time when the current
recorded jumps
to total amplitude that is twice as large as that carried when a single
channel is opened (implying that there are at least two channels in the
patch!).
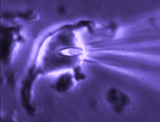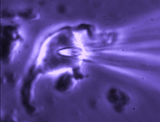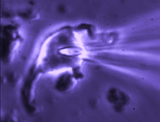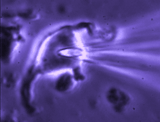 Patch clamp -whole cell recording.
Here, a gigaseal is formed as described for single channel recording.
Except that a brief pulse of current is used to destroy the small patch
of membrane that lies below the tip of the pipette. As a result, direct
electrical contact is made between the inside of the pipette and the
interior of the cell. This "whole cell" configuration allows high
quality current-clamp and voltage-clamp recordings to be made from
neurons. One advantage of this
Patch clamp -whole cell recording.
Here, a gigaseal is formed as described for single channel recording.
Except that a brief pulse of current is used to destroy the small patch
of membrane that lies below the tip of the pipette. As a result, direct
electrical contact is made between the inside of the pipette and the
interior of the cell. This "whole cell" configuration allows high
quality current-clamp and voltage-clamp recordings to be made from
neurons. One advantage of this
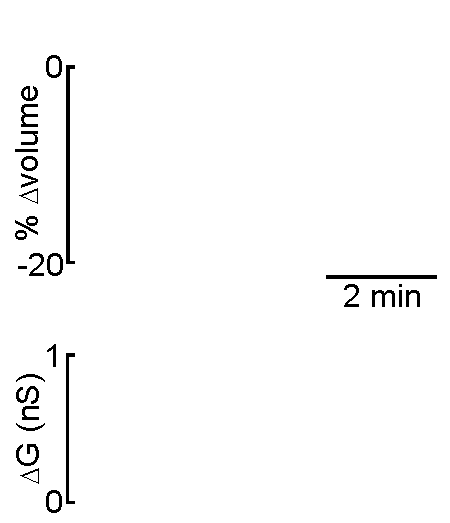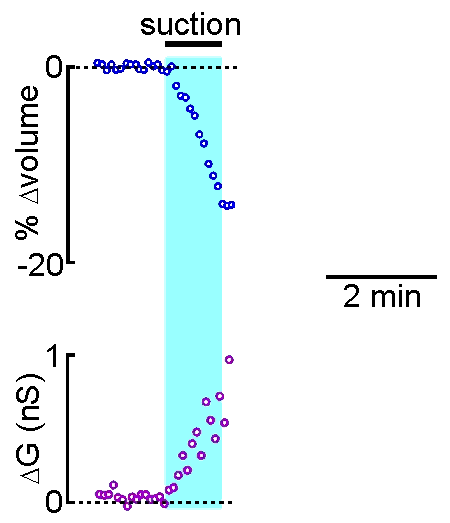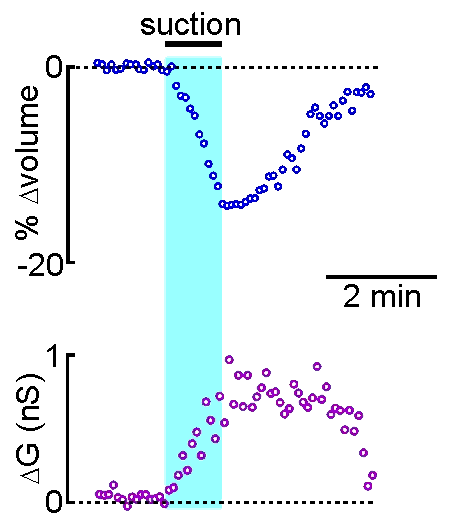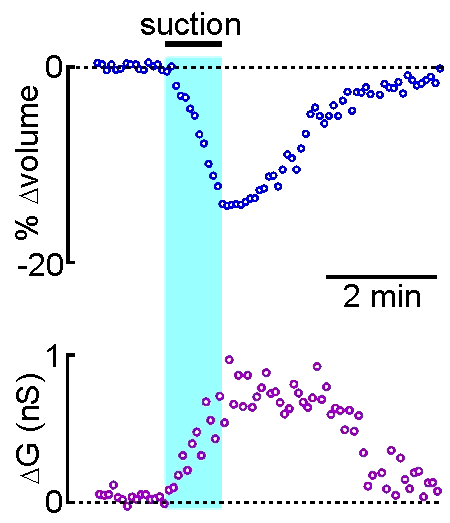 technique is that drugs, dyes or
fluorescent probes (e.g. Calcium indicators) can be easily delivered to
the interior of the cell. Because this technique is most often performed
while observing the cell with a microscope, it is easy to combine
electrophysiological experiments with simultaneous imaging. In the
example shown at right, the volume of a supraoptic nucleus neuron was reduced by
applying suction to a recording pipette. As can be seen by the graphical
representation of an equivalent experiment, the decrease in cell volume
caused by suction is associated with an increase in the membrane
conductance (G) of the cell as measured by voltage clamp. This indicates
that cell shrinking is associated with the opening of ion channels.This
finding provides insight into one of the mechanisms underlying
osmosensory transduction in these neurons.
technique is that drugs, dyes or
fluorescent probes (e.g. Calcium indicators) can be easily delivered to
the interior of the cell. Because this technique is most often performed
while observing the cell with a microscope, it is easy to combine
electrophysiological experiments with simultaneous imaging. In the
example shown at right, the volume of a supraoptic nucleus neuron was reduced by
applying suction to a recording pipette. As can be seen by the graphical
representation of an equivalent experiment, the decrease in cell volume
caused by suction is associated with an increase in the membrane
conductance (G) of the cell as measured by voltage clamp. This indicates
that cell shrinking is associated with the opening of ion channels.This
finding provides insight into one of the mechanisms underlying
osmosensory transduction in these neurons.
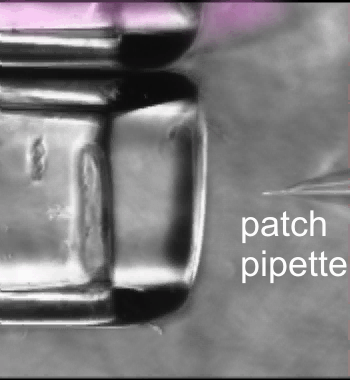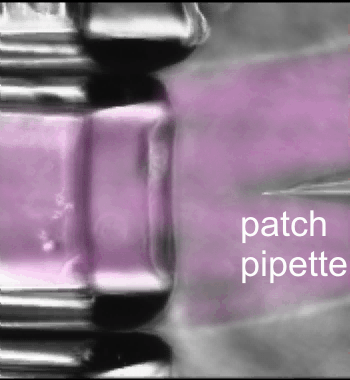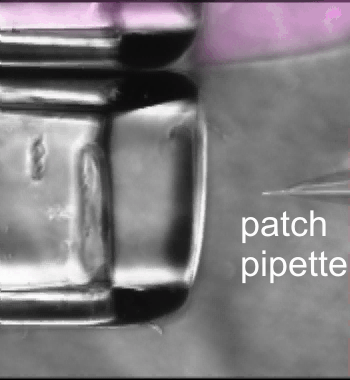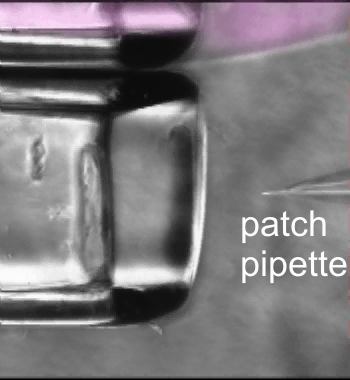 Fast
drug application. Whole cell recordings are used to record the
simultaneous activity of many ion channels, or fast responses involving
a subset of ion channels activated by fast drug delivery using a fast
stepper system or responses evoked by neurotransmitter release induced
by the activation of synaptic afferents. In the movie shown at left, a
tiny neuron (not visible) at the tip of the patch pipette is being
repeatedly exposed to either control or drug-containing
solution (purple). The solutions are delivered via an assembly of
multiple square glass pipettes (3 in this case) which is rapidly moved
using a computer-controlled piezoelectric device while the response of
the neuron is being recorded.
Fast
drug application. Whole cell recordings are used to record the
simultaneous activity of many ion channels, or fast responses involving
a subset of ion channels activated by fast drug delivery using a fast
stepper system or responses evoked by neurotransmitter release induced
by the activation of synaptic afferents. In the movie shown at left, a
tiny neuron (not visible) at the tip of the patch pipette is being
repeatedly exposed to either control or drug-containing
solution (purple). The solutions are delivered via an assembly of
multiple square glass pipettes (3 in this case) which is rapidly moved
using a computer-controlled piezoelectric device while the response of
the neuron is being recorded.
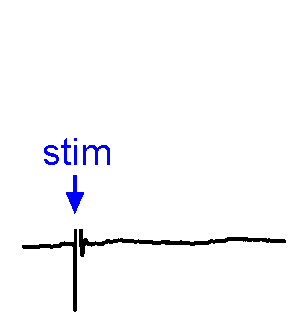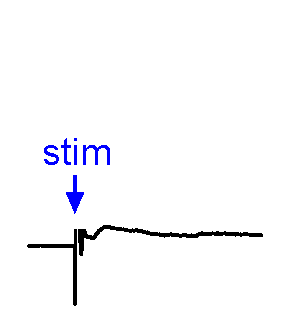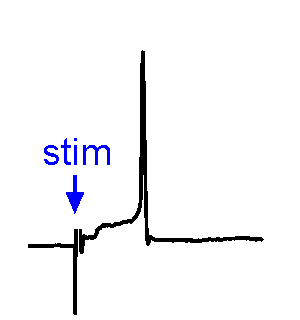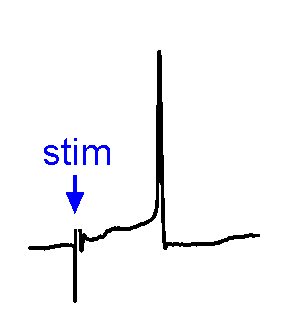
Synaptic Responses. Our research involves analysis of spontaneous and evoked synaptic responses. In the example shown at right, voltage responses are recorded in current-clamp from a supraoptic nucleus neuron while an afferent nucleus is activates by a brief (1 ms) electrical pulse (stim). In some sweeps no response occurs, while in others an excitatory postsynaptic potential (EPSP) is visible. In a few sweeps the evoked EPSP generates an action potential. We also frequently examine the effects of physiological stimulation of afferents. For example, we examine the effects of osmotic or thermal stimulation of central sensory neurons in one nucleus while recording changes in synaptic activity relayed to downstream neurons. An example of this approach is shown in the Research page.
 Cells
and tissues. Our work is performed on models that replicate
functions present in the human brain. We study acutely isolated or
cultured somata or isolated nerve terminals to define the roles of
specific types of ion channels and receptors in identified cells and
cellular compartments. Recordings in acute slices, superfused explants
or organotypic slice cultures are used to study neuron-glia
interactions, and the integration of cellular, synaptic and network
properties in-situ.
Cells
and tissues. Our work is performed on models that replicate
functions present in the human brain. We study acutely isolated or
cultured somata or isolated nerve terminals to define the roles of
specific types of ion channels and receptors in identified cells and
cellular compartments. Recordings in acute slices, superfused explants
or organotypic slice cultures are used to study neuron-glia
interactions, and the integration of cellular, synaptic and network
properties in-situ.
Optogenetics. Genetic or
 viral
approaches are used to express light-activated molecules in specific cells
(e.g. channelrhodopsin or archaerhodopsin) to define the role of
these neurons in circuits that mediate behavior and endocrine
responses. Such experiments are performed in vivo or (as done
here) in
vitro, where action potential firing is precisely suppressed by a
pulse of laser light.
viral
approaches are used to express light-activated molecules in specific cells
(e.g. channelrhodopsin or archaerhodopsin) to define the role of
these neurons in circuits that mediate behavior and endocrine
responses. Such experiments are performed in vivo or (as done
here) in
vitro, where action potential firing is precisely suppressed by a
pulse of laser light.Tissue culture and molecular biology. Our experiments also involve the use of organotypic slice cultures, cultured cell lines, in-vitro transfection of these preparations, as well as standard molecular biology techniques such as western blotting and RT-PCR for detection of specific mRNA species in single cells and tissue extracts.
Electrophysiology. The Bourque Lab is first and foremost an electrophysiology lab. Most trainees will gain experience with many different electrophysiological techniques as required by their particular project, including:
Extracellular single-unit recording. Here, a glass pipettes filled
Unlike many other techniques, electrophysiological experiments provide data and information that can be observed in real time. No need to wait an hour or a day to know if the experiment worked! The animation shown above illustrates a measurement of the neurons' V-I profile as done in real time. Evidently protocols such as this are usually performed at a slightly slower rate, and must be repeated often during an experiment. But the information they provide can be interpreted on-line.
Patch clamp - single channel recording. Here, a larger tipped (diameter 1-2 microns) glass pipette is pressed against the membrane of a neuron while it is observed using a microscope. Gentle suction is applied and the high resistance seal that forms (a so-called "gigaseal") allows one to record the activity of individual ion channel proteins as the pore opens and closes to regulate
 technique is that drugs, dyes or
fluorescent probes (e.g. Calcium indicators) can be easily delivered to
the interior of the cell. Because this technique is most often performed
while observing the cell with a microscope, it is easy to combine
electrophysiological experiments with simultaneous imaging. In the
example shown at right, the volume of a supraoptic nucleus neuron was reduced by
applying suction to a recording pipette. As can be seen by the graphical
representation of an equivalent experiment, the decrease in cell volume
caused by suction is associated with an increase in the membrane
conductance (G) of the cell as measured by voltage clamp. This indicates
that cell shrinking is associated with the opening of ion channels.This
finding provides insight into one of the mechanisms underlying
osmosensory transduction in these neurons.
technique is that drugs, dyes or
fluorescent probes (e.g. Calcium indicators) can be easily delivered to
the interior of the cell. Because this technique is most often performed
while observing the cell with a microscope, it is easy to combine
electrophysiological experiments with simultaneous imaging. In the
example shown at right, the volume of a supraoptic nucleus neuron was reduced by
applying suction to a recording pipette. As can be seen by the graphical
representation of an equivalent experiment, the decrease in cell volume
caused by suction is associated with an increase in the membrane
conductance (G) of the cell as measured by voltage clamp. This indicates
that cell shrinking is associated with the opening of ion channels.This
finding provides insight into one of the mechanisms underlying
osmosensory transduction in these neurons.Synaptic Responses. Our research involves analysis of spontaneous and evoked synaptic responses. In the example shown at right, voltage responses are recorded in current-clamp from a supraoptic nucleus neuron while an afferent nucleus is activates by a brief (1 ms) electrical pulse (stim). In some sweeps no response occurs, while in others an excitatory postsynaptic potential (EPSP) is visible. In a few sweeps the evoked EPSP generates an action potential. We also frequently examine the effects of physiological stimulation of afferents. For example, we examine the effects of osmotic or thermal stimulation of central sensory neurons in one nucleus while recording changes in synaptic activity relayed to downstream neurons. An example of this approach is shown in the Research page.